Aspirin-Mediated Acetylation of SIRT1 Maintains Intestinal Immune Homeostasis
Liangguo Xie, Chaoqun Li, Chao Wang, Zhen Wu, Changchun Wang, Chunyu Chen, Xiaojian Chen, Dejian Zhou, Qiang Zhou, Ping Lu, Chen Ding, Chen-Ying Liu, Jinzhong Lin, Xumin Zhang, Xiaofei Yu,* Wei Yu*
Adv. Sci. 2024, 2306378.
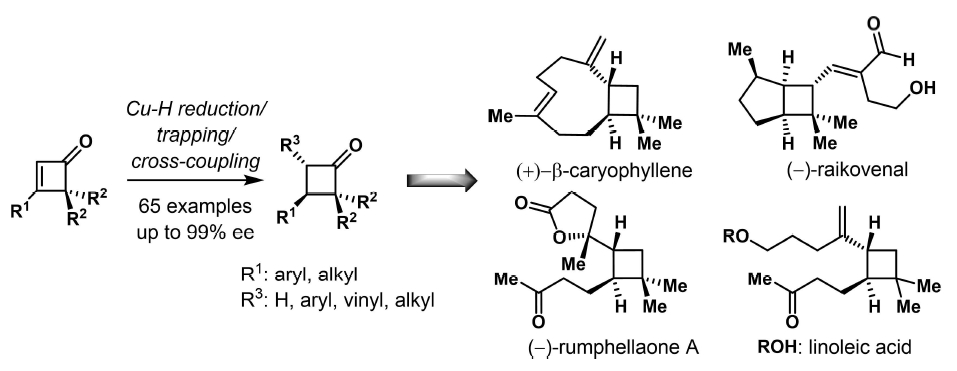
Palladium-Catalyzed Divergent Enantioselective Functionalization of Cyclobutenes
Zhonggui Wang,§ Jie Zhu,§ Minyan Wang,* and Ping Lu*
J. Am. Chem. Soc., 2024, 10.1021/jacs.4c02215.
.png)
Shaowei Wang+, Changxu Zhong+, Yingchao Huang, and Ping Lu*
Angew. Chem. Int. Ed. 2024, e202400515.
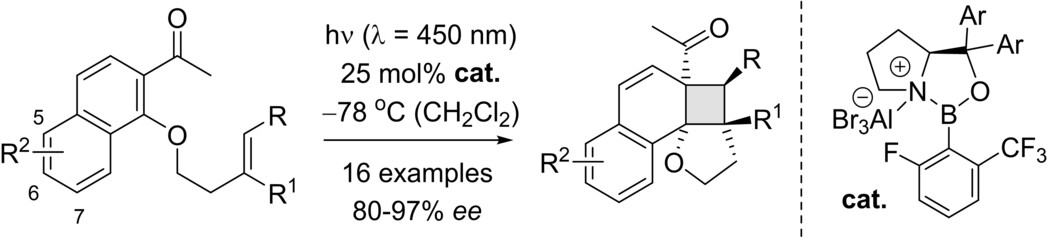
Enantioselective Intramolecular ortho Photocycloaddition Reactions of 2-Acetonaphthones
Peng Yan, Simone Stegbauer, Qinqin Wu, Elena Kolodzeiski, Christopher J. Stein, Ping Lu,* and Thorsten Bach*
Angew. Chem. Int. Ed. 2024, 63, e202318126.
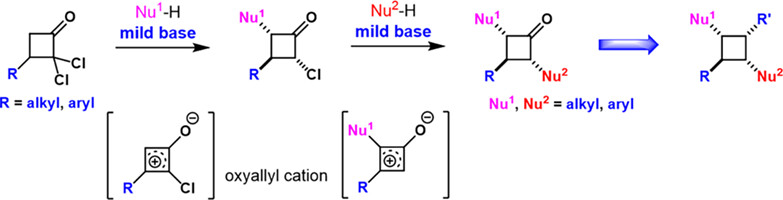
Modular Synthesis of Multi substituted Cyclobutanones Enabled by Oxyallyl Cations
Meng Wang, Zhonggui Wang, and Ping Lu*
Chin. J. Chem. 2024, 42, 459–463. (Dedicated to the Memory of Professor Xiyan Lu)
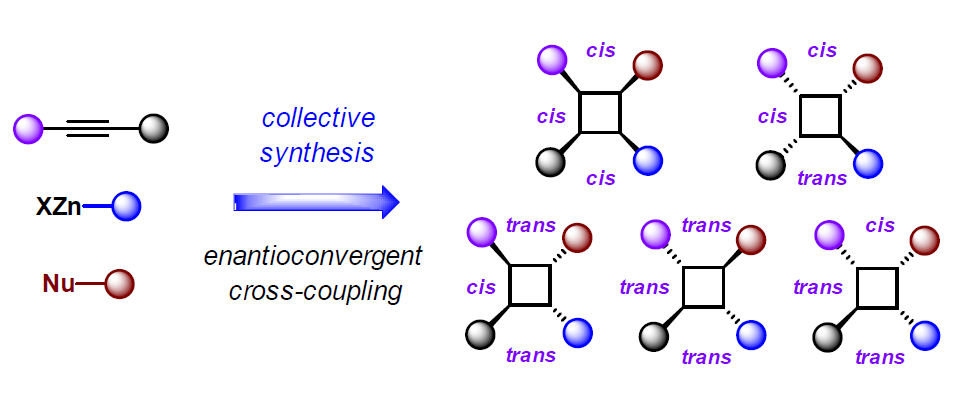
Collective Synthesis of Chiral Tetrasubstituted Cyclobutanes Enabled by Enantioconvergent Negishi Cross-Coupling of Cyclobutenones
Min Yan+, Qiang Zhou+, and Ping Lu*
Angew. Chem. Int. Ed. 2023, 62, e202218008.
Enantioselective [3+2]-cycloaddition of 2,3-disubstituted cyclobutenones: vicinal quaternary stereocenters construction and skeletal functionalization
Licheng Lu, and Ping Lu*
Chem. Sci., 2023, 14, 8355–8359
Enantioselective Construction of Vicinal Angular Quaternary Stereocenters Enabled by Strained Cyclobutenones
Peng Yan, Jia Zhang, Licheng Lu, Huayi Fang, and Ping Lu*
ACS Catal. 2022, 12, 15416−15423
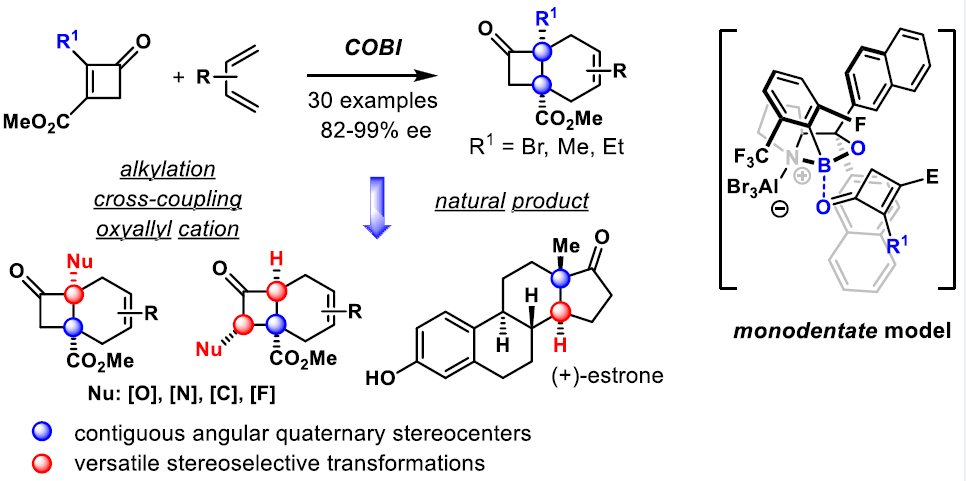
Diastereoselective synthesis of 1,1,3,3-tetrasubstituted cyclobutanes enabled by cycloaddition of bicyclo[1.1.0]butanes
Meng Wang, Yingchao Huang, Chunyu Li, and Ping Lu*
Org. Chem. Front., 2022, 9, 2149–2153.
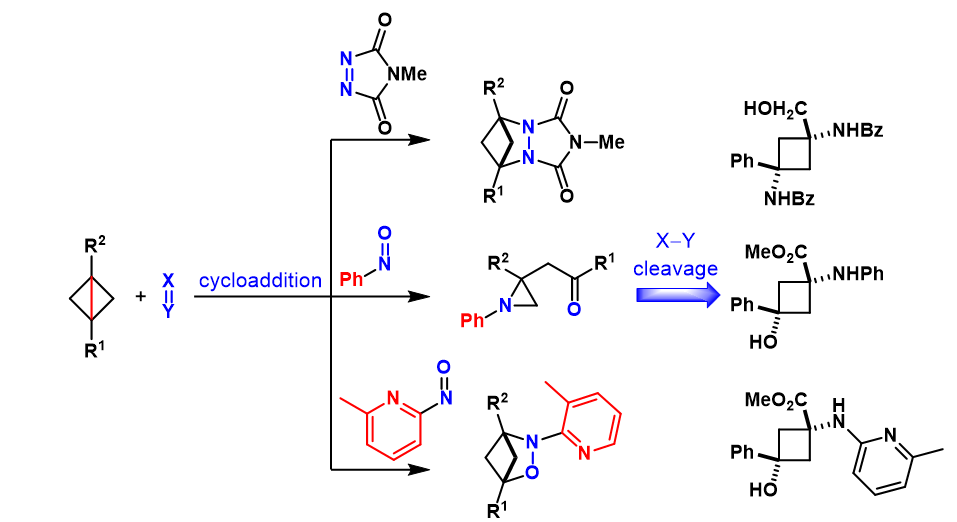
Total Synthesis of (+)-Kingianin A by Enantioselective Cycloaddition of Strained Cyclobutenone
Jie Zhanga, Peng Yanb,ZhichaoWangb, Jinbo Zhao*a, Qin Chen*b, Ping Lu*b
Synthesis 2022, 54, 1977–1982. (Invited Feature Article)
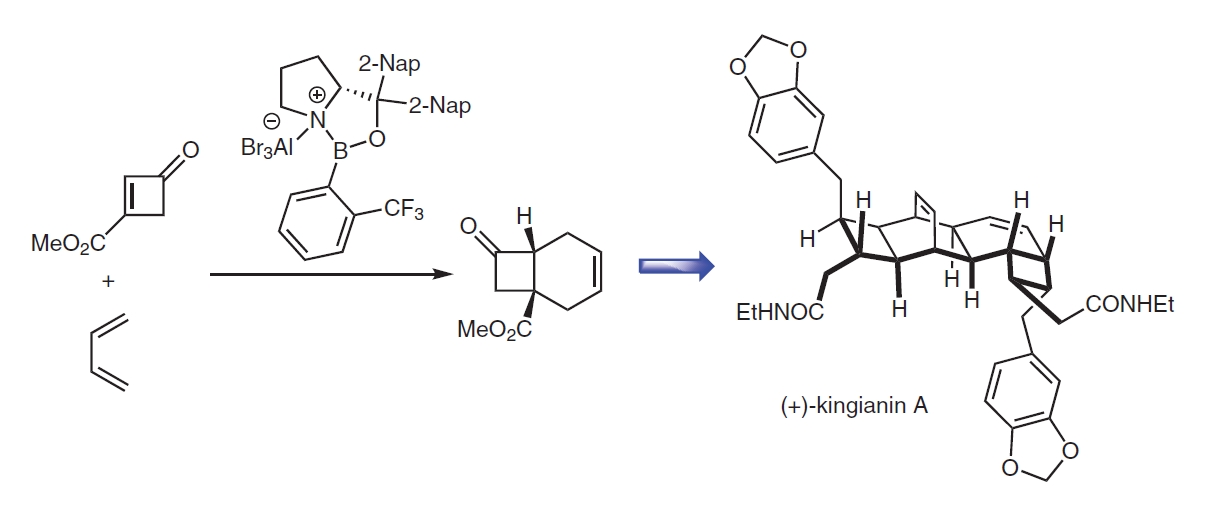
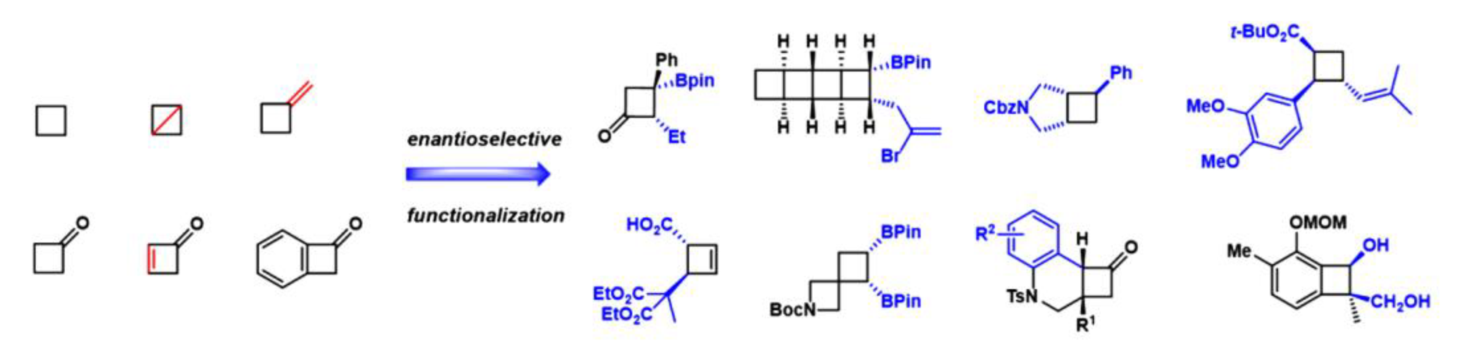
Dancing on Ropes Enantioselective Functionalization of Preformed Four Membered Carbocycles
Jun Chen, Qiang Zhou, Huayi Fang,* and Ping Lu*
Chin. J. Chem. 2022, 40, 1346–1358.
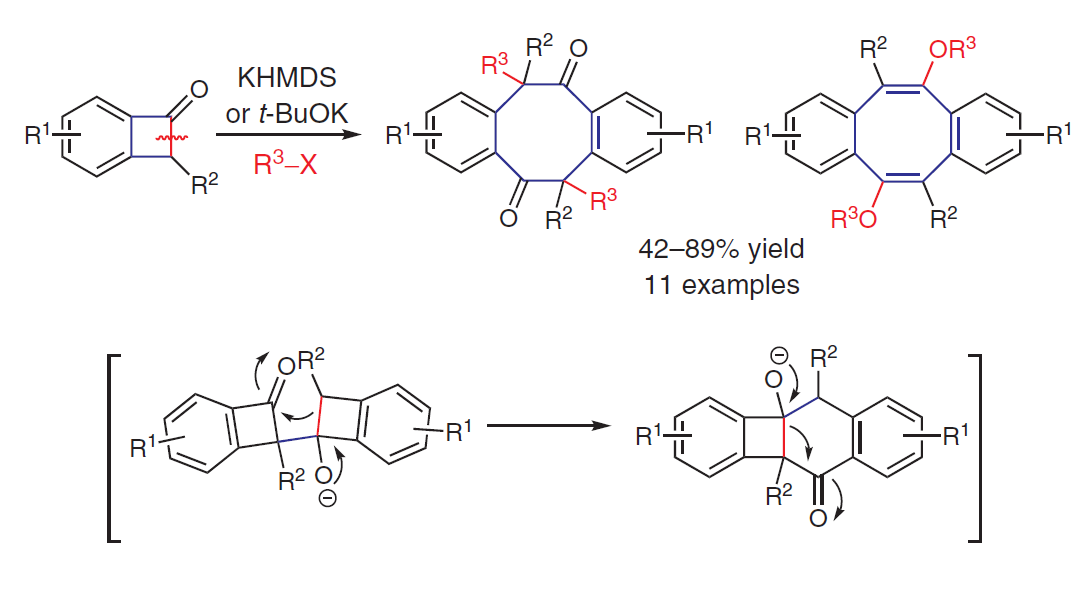
Synthesis of Dibenzo[a,e]cyclooctene-5,11(6H,12H)-diones via the Elusive Benzocyclobutenone Anion
Yingchao Huang, Jun Chen, Yu Liu*, and Ping Lu*
Synthesis 2021, 53, 4477–4483.
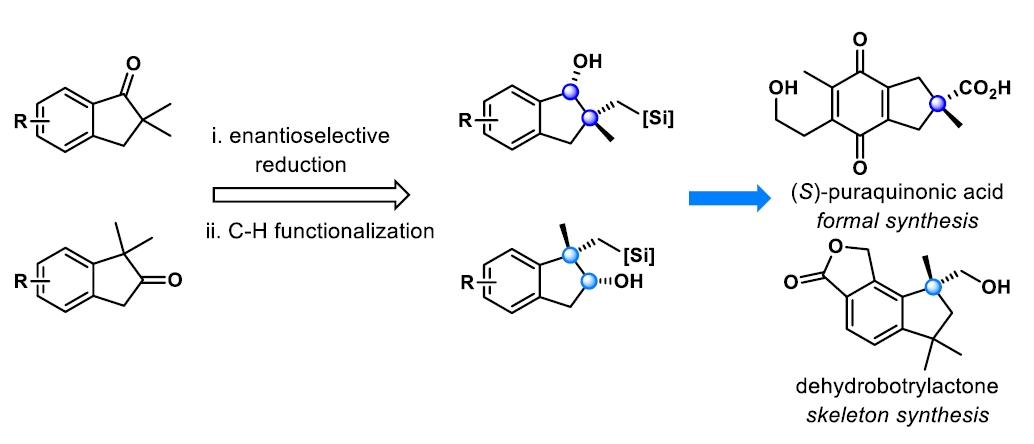
Enantioselective Synthesis of Indanes with a Quaternary Stereocenter via Diastereoselective C(sp3)−H Functionalization
Jun Chen, Zhan Shi, and Ping Lu*
Org. Lett. 2021, 23, 7359−7363.
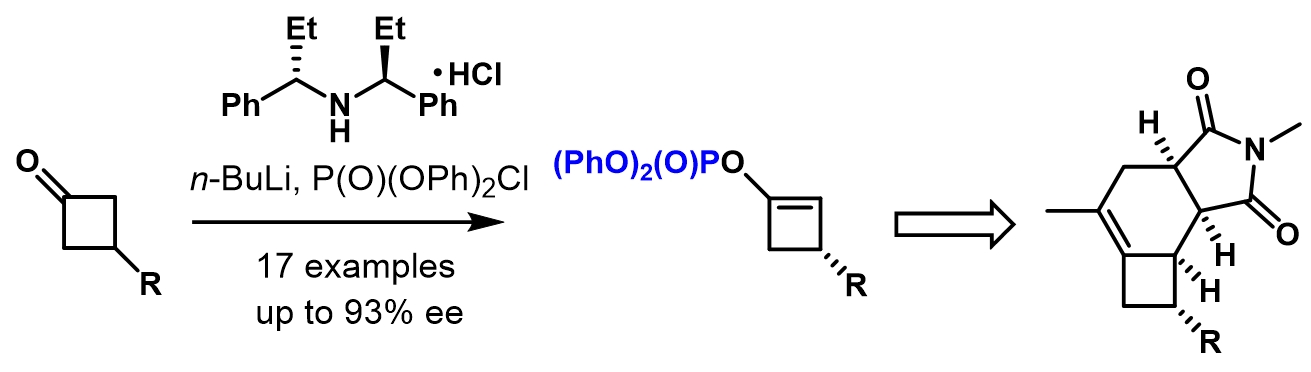
Peng Yan, Changxu Zhong, Jie Zhang, Yu Liu, Huayi Fang, and Ping Lu*
Angew. Chem. Int. Ed. 2021, 60, 4609–4613.

Enantioselective Functionalization of Prochiral Cyclobutanones and Cyclobutenones
Meng Wang, Changxu Zhong, and Ping Lu*
Synlett, 2021, 32, 1253–1259. (Invited Synpacts)

Jun Chen, Zhan Shi, Chunyu Li. and Ping Lu*
Chem. Sci. 2021, 12, 10598-10604。
Chiral lithium amide mediated desymmetrization of 3-substituted cyclobutanone
Changxu Zhong, Shaowei Wang, and Ping Lu*
Org. Chem. Front., 2021, 8, 2977–2980.



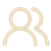

.png)

.png)

 loading......
loading......